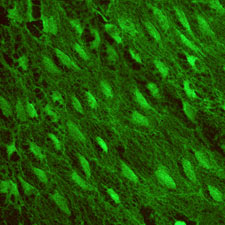
 One
major focus of study in the
Gimbrone laboratory has been the molecular mechanisms that mediate
the localized interactions of leukocytes with the vascular endothelium
at sites of acute and chronic inflammatory responses, and vascular
injury and repair. Our working concept has been that endothelium-dependent
mechanisms (in particular, inducible cell surface adhesion molecules
and secreted cytokines/chemokines, such as IL-8 and MCP-1) are
important local determinants of the spatial and temporal patterns
of leukocyte-vessel wall interactions. Research accomplishments
in this area include: 1) the demonstration in the early-mid
1980's, with colleagues in the Vascular Research Division, of
the phenomenon of "endothelial activation" by proinflammatory
cytokines and bacterial products (e.g., Gram-negative endotoxins);
2) the discovery of cytokine-inducible endothelial-leukocyte
adhesions molecules (ELAMs), and the molecular cloning of ELAM-1
(E-selectin), the index member of a novel family of intercellular
adhesion molecules called the "Selectins"; 3) the
molecular cloning of a novel endothelial isoform of IL-8 and
the demonstration of its role, in vitro and in vivo, as an endothelium-derived
soluble inhibitor of leukocyte adhesion; 4) the discovery and
molecular cloning of "ATHERO-ELAM", a mononuclear
leukocyte-selective adhesion molecule that is expressed by the
endothelium in the earliest atherosclerotic lesions in hypercholesterolemic
preclinical models, and the demonstration of
its homology with human VCAM-1 (vascular cell adhesion molecule-1);
5) the engineering (in collaboration with Dr. David Milstone
in the Vascular Research Division) of an E-selectin-deficit
mouse, which has been useful for various pathophysiological
studies; 6) the development of techniques (in collaboration
with the Vascular Research Division Cell Biology Core) for isolation
and culture of microvascular endothelial cells from the lungs
of genetically modified mice, for use in in vitro studies. Ongoing
projects include: 1) detailed structure-function
studies of the cytoplasmic domain of E-selectin and its role
in transmembrane signalling during inflammatory leukocyte recruitment;
2) downstream consequences of E-selectin-mediated, leukocyte-adhesion-dependent
signalling, in particular the modulation of endothelial phenotype,
at the transcriptional level; 3) immunotargeting to cell surface
endothelial activation antigens, such as E-selectin and VCAM-1,
for diagnostic and therapeutic applications including vascular
imaging and gene transfer.
One
major focus of study in the
Gimbrone laboratory has been the molecular mechanisms that mediate
the localized interactions of leukocytes with the vascular endothelium
at sites of acute and chronic inflammatory responses, and vascular
injury and repair. Our working concept has been that endothelium-dependent
mechanisms (in particular, inducible cell surface adhesion molecules
and secreted cytokines/chemokines, such as IL-8 and MCP-1) are
important local determinants of the spatial and temporal patterns
of leukocyte-vessel wall interactions. Research accomplishments
in this area include: 1) the demonstration in the early-mid
1980's, with colleagues in the Vascular Research Division, of
the phenomenon of "endothelial activation" by proinflammatory
cytokines and bacterial products (e.g., Gram-negative endotoxins);
2) the discovery of cytokine-inducible endothelial-leukocyte
adhesions molecules (ELAMs), and the molecular cloning of ELAM-1
(E-selectin), the index member of a novel family of intercellular
adhesion molecules called the "Selectins"; 3) the
molecular cloning of a novel endothelial isoform of IL-8 and
the demonstration of its role, in vitro and in vivo, as an endothelium-derived
soluble inhibitor of leukocyte adhesion; 4) the discovery and
molecular cloning of "ATHERO-ELAM", a mononuclear
leukocyte-selective adhesion molecule that is expressed by the
endothelium in the earliest atherosclerotic lesions in hypercholesterolemic
preclinical models, and the demonstration of
its homology with human VCAM-1 (vascular cell adhesion molecule-1);
5) the engineering (in collaboration with Dr. David Milstone
in the Vascular Research Division) of an E-selectin-deficit
mouse, which has been useful for various pathophysiological
studies; 6) the development of techniques (in collaboration
with the Vascular Research Division Cell Biology Core) for isolation
and culture of microvascular endothelial cells from the lungs
of genetically modified mice, for use in in vitro studies. Ongoing
projects include: 1) detailed structure-function
studies of the cytoplasmic domain of E-selectin and its role
in transmembrane signalling during inflammatory leukocyte recruitment;
2) downstream consequences of E-selectin-mediated, leukocyte-adhesion-dependent
signalling, in particular the modulation of endothelial phenotype,
at the transcriptional level; 3) immunotargeting to cell surface
endothelial activation antigens, such as E-selectin and VCAM-1,
for diagnostic and therapeutic applications including vascular
imaging and gene transfer.
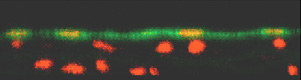 A
second major focus of activity
in the Gimbrone Laboratory (which has evolved from a long-standing
collaboration with Prof. C.F. Dewey and colleagues in the Fluid
Mechanics Laboratory at the Massachusetts Institute of Technology)
is the study of hemodynamic forces, such as wall shear stress,
as modulators of vascular endothelial structure and function.
Specially designed in vitro flow devices are used to expose
cultured endothelial monolayers to defined laminar, disturbed
laminar, and turbulent flow regimens, and the resultant morphological,
biochemical and molecular genetic changes are studied in the
context of vascular adaptation, and also the pathogenesis of
vascular diseases, in particular atherosclerosis. Research
contributions in this area include: 1) the
demonstration that shear stresses can differentially regulate
important aspects of endothelial cell biology, including cell
shape and cytoskeletal organization, cell growth and apoptosis,
cell endocytosis and secretion, and gene expression; 2) the
discovery and characterization of "shear stress response
elements (SSREs)" in the promoters of certain biologically
important, endothelial-expressed genes, that mediate their induction
by biomechanical forces; 3) the demonstration, by high-through-put
genomic strategies (such as differential display of expressed
transcripts and cDNA microarrays), that endothelial gene expression
can be profoundly influenced by different types of biomechanical
input stimuli; 4) the demonstration that the biomechanical milieu
of so-called atherosclerosis-resistant arterial geometries may
favor the sustained upregulation of critical "athero-protective
genes" in the endothelium (the "Athero-protective
Gene Hypothesis"); 5) the discovery of several novel genes
(e.g., Smad 6, Smad 7) whose expression in endothelium is biomechanically
regulated and potentially relevant to vascular homeostasis.
Ongoing studies include: 1) in vitro modeling
of the effects of dynamic (arterial waveform) flow patterns
on endothelial functional phenotype; 2) in-depth analyses of
the patterns of gene expression induced by different biomechanical
forces in cultured endothelial cells, utilizing genome-wide
transcriptional profiling strategies; 3) extension of these
studies to the endothelial lining of vessels in vivo, in both
animal models and human vascular specimens (normal and diseased).
A
second major focus of activity
in the Gimbrone Laboratory (which has evolved from a long-standing
collaboration with Prof. C.F. Dewey and colleagues in the Fluid
Mechanics Laboratory at the Massachusetts Institute of Technology)
is the study of hemodynamic forces, such as wall shear stress,
as modulators of vascular endothelial structure and function.
Specially designed in vitro flow devices are used to expose
cultured endothelial monolayers to defined laminar, disturbed
laminar, and turbulent flow regimens, and the resultant morphological,
biochemical and molecular genetic changes are studied in the
context of vascular adaptation, and also the pathogenesis of
vascular diseases, in particular atherosclerosis. Research
contributions in this area include: 1) the
demonstration that shear stresses can differentially regulate
important aspects of endothelial cell biology, including cell
shape and cytoskeletal organization, cell growth and apoptosis,
cell endocytosis and secretion, and gene expression; 2) the
discovery and characterization of "shear stress response
elements (SSREs)" in the promoters of certain biologically
important, endothelial-expressed genes, that mediate their induction
by biomechanical forces; 3) the demonstration, by high-through-put
genomic strategies (such as differential display of expressed
transcripts and cDNA microarrays), that endothelial gene expression
can be profoundly influenced by different types of biomechanical
input stimuli; 4) the demonstration that the biomechanical milieu
of so-called atherosclerosis-resistant arterial geometries may
favor the sustained upregulation of critical "athero-protective
genes" in the endothelium (the "Athero-protective
Gene Hypothesis"); 5) the discovery of several novel genes
(e.g., Smad 6, Smad 7) whose expression in endothelium is biomechanically
regulated and potentially relevant to vascular homeostasis.
Ongoing studies include: 1) in vitro modeling
of the effects of dynamic (arterial waveform) flow patterns
on endothelial functional phenotype; 2) in-depth analyses of
the patterns of gene expression induced by different biomechanical
forces in cultured endothelial cells, utilizing genome-wide
transcriptional profiling strategies; 3) extension of these
studies to the endothelial lining of vessels in vivo, in both
animal models and human vascular specimens (normal and diseased).
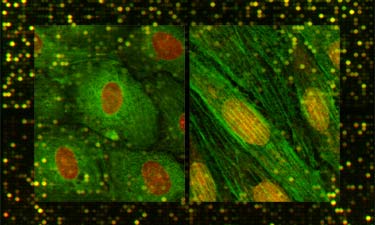 Finally,
the Gimbrone Laboratory has undertaken a systematic approach
to defining phenotypic modulation of vascular endothelium, in
health and disease, utilizing state-of-the-art techniques for
transcriptional
profiling of endothelial gene expression, in vitro and
in vivo. It is our intent that the experimental databases resulting
from these studies will be made available via this website for
use by the vascular biology community.
Finally,
the Gimbrone Laboratory has undertaken a systematic approach
to defining phenotypic modulation of vascular endothelium, in
health and disease, utilizing state-of-the-art techniques for
transcriptional
profiling of endothelial gene expression, in vitro and
in vivo. It is our intent that the experimental databases resulting
from these studies will be made available via this website for
use by the vascular biology community.
home
| projects | members
| publications
| links
transcriptional
profiling project | vascular
research division